Cell quality control
The glands produce and transport substances essential for the coordinated functioning of the human and animal body to the right places, where they are used as quickly as possible. Examples include digestive enzymes produced by the salivary glands, pancreas, stomach and small intestine, and insulin produced by special cells in the islets of Langerhans.
These special cells, however, only excrete part of the secretions produced, the residue, packaged in secretory granules, remains in the cytoplasm of the cells, where it is fused with cellular vesicles (lysosomes) containing digestive enzymes and then broken down. Little is known about this process, known as crinophagy, although its biomedical importance is considerable.
Crinophagy not only removes secretory particles remaining in the cytoplasm, but also removes poor quality, abnormally formed granules, thus preventing their potentially defective contents from being discharged from the cell. In the newly published work, it was discovered that the degradation process can take place at the early stage of development of the larval salivary gland of Drosophila. Now that we know the entire life cycle of the secretory granules, we can interfere with this process.
The researchers used genetic techniques to prevent the growth of these secretory granules and found that the cell did not shed these young, immature granules, but instead diverted them to the degradation pathway. Therefore this is a developmental program-independent secretory granule degradation (crinophagy), activity that contributes to our understanding of the relationship between the formation, maturation and the emptying and degradation of secretory particles. The combination of all these processes contributes to the normal functioning of glandular cells and thus to the maintenance of our health.
In 2018, a group of researchers at the Department of Anatomy, Cell and Developmental Biology at Eötvös Loránd University (ELTE), led by Tamás Csizmadia, discovered the first genes that regulate this developmental program in the glandular cell. More recently, the researchers have been investigating how secretory granules, which are used by glandular cells to store secretions within the cell, are broken down.
Salivary glands from larvae and young pupae of Drosophila were used for the study.
"The cells of Drosophila function very similarly to those of humans. Researchers have already mapped the genomes of Drosophila and humans (i.e. the set of hereditary information encoded in DNA), but the genetic make-up of this small fly is much simpler and therefore easier to observe. If we can understand which genes regulate the function of Drosophila cells, we can understand the basic cellular biology of humans," says Tamás Csizmadia, first author of the paper presenting the new research.
"Since we know the entire life cycle of the secretory granule, we thought we could intervene in this process. After using genetic methods to prevent the growth of the secretory particles, we found that the cell did not shed the young, immature granules, but rather put them on the pathway to degradation," explains Péter Lőw, assistant professor and final author of the study.
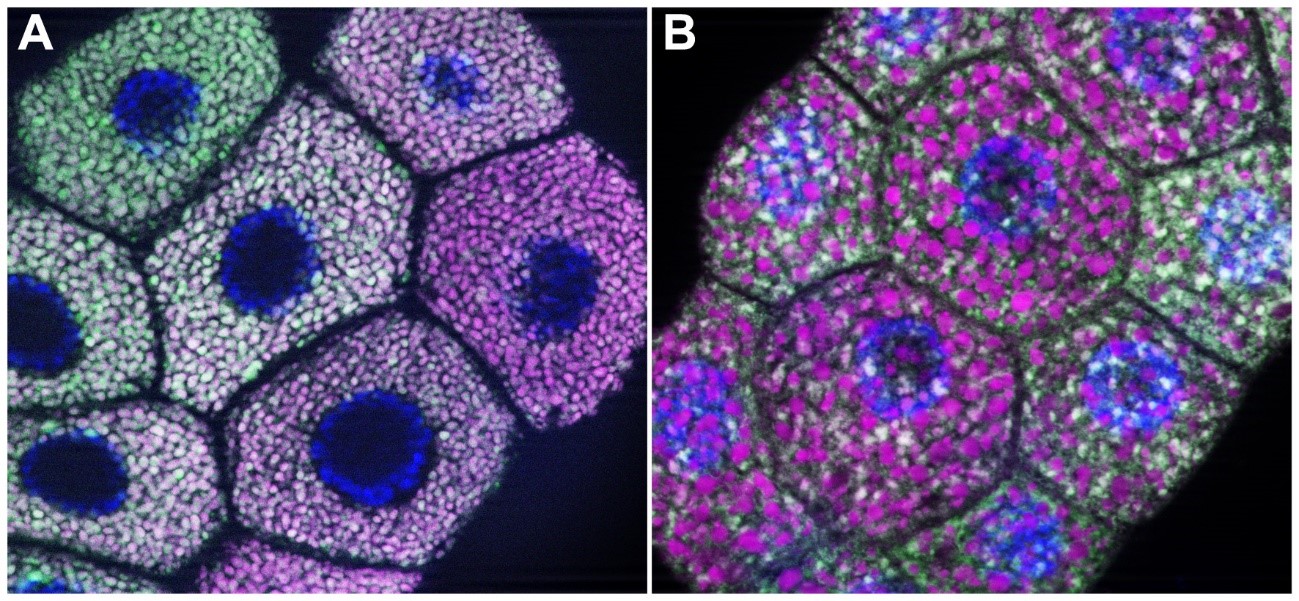
In their experiment, the researchers labelled the secretory granules of Drosophila salivary gland cells both green and magenta. Normally, the secretory granules are of the right size, are well separated from each other and contain both colours (image A). After the researchers knocked out the gene necessary for secretory granule maturation (image B), the secretory granules failed to reach their normal size, i.e. they were not of the right quality (the greenish-white spots in image B) and were prematurely diverted to the degradation pathway (the larger magenta spots in image B). In the figure, the nuclei are marked in blue.
The researchers have therefore discovered that there is also a developmental program-independent pathway of secretory granule degradation that occurs when there is a disruption in the early stages, maturation and growth of the secretory granules. In other words, the role of crinophagy is not only to degrade the excess secretory granules that remain in the cell during development, but also appears to be to control the quality of secretory granules at a very early stage of development.
The result is essential for understanding the relationship between the maturation, growth, secretion and breakdown of secretory particles in glandular cells. Together, these processes contribute to the healthy, or normal, functioning of glandular cells.
For example, a significant part of our pancreas produces initially inactive digestive enzymes that are activated during elimination in the duodenum. However, if these secretory particles cannot be eliminated (for example, due to gallstones), they are diverted to a degradation pathway within the cell, where they are activated locally and then released from the cell, causing the death of the glandular cells. This is how acute pancreatitis, for example, can develop. Therefore, it is of utmost importance to prevent increased intracellular degradation of secretory particles in these cells.
This can only be done by identifying the molecules that regulate and drive this process. In other words, understanding how our cells work and knowing exactly what goes wrong in them, what leads to disease, can give medicine the tools, such as drug development, to help us fix the problems within cells. It is important to know that Drosophila cells function in a very similar way to human cells, making them an excellent model organism for genetic and cell biology research.
The research report was published in the journal Traffic in November 2022. A unique feature of the work is that three of the seven authors are currently undergraduate students in biology and one is a Master's student in biology. The Biological Research Centre (BRC) in Szeged also made a significant contribution to the research.
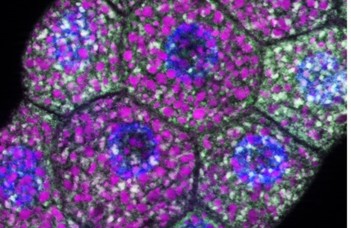
Cover photo: Early degradation of secretory granules in Drosophila larval salivary gland cells (photo by Tamás Csizmadia)

